Shop with confidence
Explore related items
Cleanroom Training - GMP Training - Auditing Sterile Production Areas - MV-MTQ-0684
Without question, the most critical areas in any plant are where sterile product are made, often under aseptic conditions. These are areas which the auditor must address in considerable detail, starting with the environment and how it is monitored. Obviously operating practices and how they are observed are critical, as are personnel matters and equipment and its cleaning. All of which must be satisfied during the audit
GMP TRAINING - AUDITING STERILE PRODUCTION AREAS INCLUDES
Access to aseptic manufacturing areas
General overview of the facility
Air filtration and pressure differentials
Requirements for areas within aseptic manufacturing
Equipment for aseptic manufacture
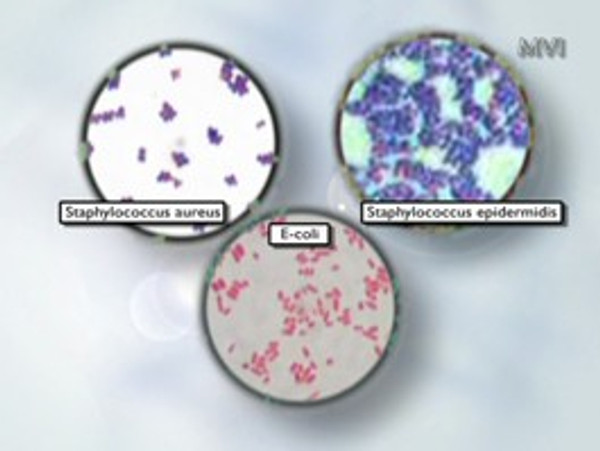
Monitoring of aseptic areas
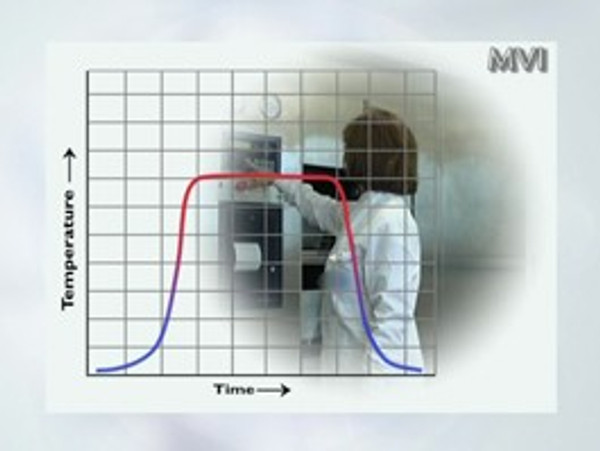
Validation
Personnel
Filtration
Testing
Video Run Time: 25 minutes
Network Licensed Video File
This format includes an optimized digital video file (MPG4) for use on a computer network. There is an accompanying HTML5 menu page and assessment. The accompanying license permits simultaneous network viewing at one named facility. Larger networks available on request.
Product Code: MV-MTQ-0684 Cleanroom Training, GMP Training, Auditing Sterile Production Areas
For more options, visit our main section for GMP training videos for cleanrooms and labs.